
What would you like to know?

Handok Pharmaceuticals is dedicated
to improving human health and the quality of life.
to improving human health and the quality of life.
Total of 234 news items
-
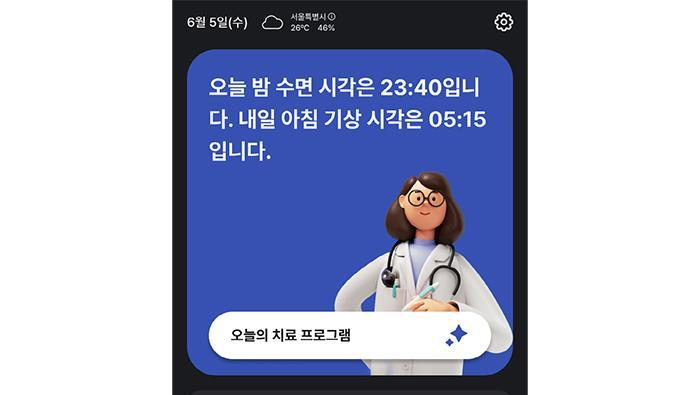 SleepQ, Digital Therapeutic for Insomnia, Achieves ISO 27001 Certification for Information Security 2025.12.19
SleepQ, Digital Therapeutic for Insomnia, Achieves ISO 27001 Certification for Information Security 2025.12.19 -
 Handok Wins Minister of Science and ICT Award as Top Project in 2025 MyData Infrastructure Program 2025.12.18
Handok Wins Minister of Science and ICT Award as Top Project in 2025 MyData Infrastructure Program 2025.12.18 -
 Handok Launches ‘Ketotop Active Plaster’ with Enhanced Drug Penetration and Cooling Effect 2025.09.18
Handok Launches ‘Ketotop Active Plaster’ with Enhanced Drug Penetration and Cooling Effect 2025.09.18 -
 Handok Launches ‘Barozen Fit Professional’ Continuous Glucose Monitoring System 2025.09.09
Handok Launches ‘Barozen Fit Professional’ Continuous Glucose Monitoring System 2025.09.09 -
 Rezolute Reaches Agreement with FDA to Streamline Phase 3 Trial Design for Tumor-Mediated Hyperinsulinism Therapy RZ358 2025.09.03
Rezolute Reaches Agreement with FDA to Streamline Phase 3 Trial Design for Tumor-Mediated Hyperinsulinism Therapy RZ358 2025.09.03 -
 Handok Manufacturing Plant Maintains Green Company Status for 28 Consecutive Years Following Renewal 2025.09.02
Handok Manufacturing Plant Maintains Green Company Status for 28 Consecutive Years Following Renewal 2025.09.02 -
 Handok’s Open Innovation R&D Achievements with ABL Bio Begin to Materialize 2025.08.14
Handok’s Open Innovation R&D Achievements with ABL Bio Begin to Materialize 2025.08.14 -
 Handok’s ‘Handok Archive 70’ Wins Publication Category at IPRA Golden World Awards 2025 2025.07.03
Handok’s ‘Handok Archive 70’ Wins Publication Category at IPRA Golden World Awards 2025 2025.07.03 -
 Handok Selected for 2025 Autonomous Factory Support Project to Strengthen Smart Manufacturing Competitiveness 2025.06.26
Handok Selected for 2025 Autonomous Factory Support Project to Strengthen Smart Manufacturing Competitiveness 2025.06.26






